
Hey, Matty Boy! You know what science you almost never talk about?
Well, hypothetical question asker, I guess I'd say chemistry.
Why is that, Matty Boy?
The main reason is that a barely know bupkiss about it.
I do know a thing or two. One thing is carbon and the other is hydrogen, the atoms you put together to form hydrocarbons. Carbon has four bonds and hydrogen only one and while sometimes the atoms are standoffish and like to keep to themselves, often they get together and fill up their entire dance cards, so every bond has two atoms. This picture of a carbon atom linked to four hydrogen atoms would make the chemical compound CH4, known by the name methane, a very common gas, known to be colorless, flammable and stinky, though the super stinky varieties owe that special ability to sulfur hanging around with them, trying to be cool and getting into the gang, even though they are just hangers on.

Another possibility is to have two carbon atoms linked and six hydrogen atoms filling in the rest of the bonds, making the chemical compound ethane, C2H6. The prefixes of hydrocarbons tell you how many carbon atoms are in the picture, where meth- means one, eth- means two, prop- means three and but- means four. The higher numbers of carbon molecules have prefixes that sound more like math prefixes, pent- for five, hex- for six, hept for seven, etc.
The two dimensional diagram as shown here is pretty standard, but the bigger illustration shown for methane is a little more accurate. The four bonds for carbon don't line up like a cross, but instead make a three dimensional shape like the corners of a tetrahedron, the simplest of the platonic solids.
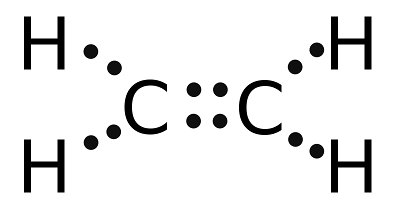
Because carbon has four bonds in a special shape, it is possible for two carbon atoms to double bond, linked to each other using two bonds apiece. Instead of two tetrahedra linked at a single corner, now they would be linked along an edge. This would mean each carbon atom has two free bonds and if all four attract hydrogen atoms, we get C2H4, known by the name ethylene.
Now we are cooking with ethyl!

Because of the geometry of it, it's impossible for two carbon atoms to bond with each other using all four bonds, but a triple bond is possible, just in the same way one face of a tetrahedron can match up with a face of a different tetrahedron. If we get carbon atoms triple bonding, then there are only two open places to bond, and if those are filled with hydrogen we get C2H2, also known as acetylene. I nicked this picture of a model of acetylene from www.pixmac.com, a company that makes cool looking chemistry models.
As I said before, I don't know much about chemistry, and if I have any spare time and cash in the future, I think I'll take a class and see if I can do something about it. I love this stuff for the mathiness of it, the geometry and graph theory, but it would be interesting to understand the properties of the hydrocarbons better than just simple classification.
Tidak ada komentar:
Posting Komentar